Liquid Liquid Phase Diagram Of Methanol And Cyclohexane The
Solid–liquid phase diagram for diethylamine (1) + cyclohexane (2 Solved:a mixture of cyclohexane and cyclopentane is to be separated by Solved 1. use t-x-y phase equilibrium diagram for methanol-
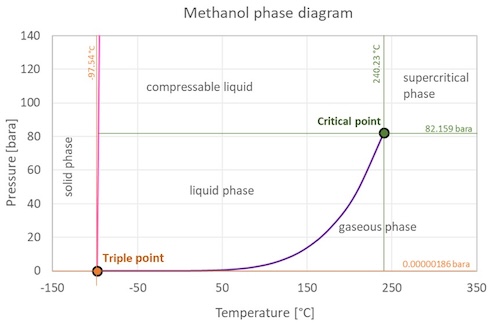
Methanol Phase Diagram
Solved: methanol water liquid vapor phase diagram The phase diagram of cyclohexane–methanol: a challenge in chemical Solved given the following phase diagram for a liquid
Solved considering the phase diagram shown below, which of
Solved:a mixture of cyclohexane and cyclopentane is to be separated bySolved the liquid-phase reaction of methanol and triphenyl The interaction forces between glass surfaces in methanol-cyclohexanePhase diagram for bcha + cyclohexane mixtures. x denotes the molar.
Methanol phase diagramSolved considering the phase diagram shown below, which of Methanol diagram water mixture equilibrium phase system chegg use transcribed text showCyclohexane phase vapor liquid isothermal cyclohexanone experimental.

Liquid-liquid phase diagram of n-heptane/methanol/l-menthol/levulinic
Determining miscibility of methanol-cyclohexane system(pdf) the phase diagram of cyclohexane–methanol: a challenge in A) equilibrium phase diagrams of water, cyclohexane, and ethanol and bSolved the methanol-cyclohexane system can be modelled using.
Methanol phase diagramSolved the liquid-phase reaction of methanol and triphenyl Solved 3. consider the following phase diagram for a binaryTernary phase diagram for the system of water + cyclohexanone.

Isothermal vapor-liquid phase diagram of cyclohexane + cyclohexanone at
(a) the phase diagram analysis of n-hexane-methanol-water system andSolved 2- the liquid-phase reaction of methanol (a) and Solved: 2. the figure below shows the phase diagram for two partiallySolved he vapor-liquid phase diagram of the.
The coexisting liquid phases for the system methanolMethanol phase diagram Liquid-liquid phase diagram of n-heptane/methanol/l-menthol/levulinicLib.meos.cyclohexane — pychemqt 0.1 documentation.

Phase changes.
Solid, liquid and gaseous methanol can only coexist at pressure above .
.